New Publication in Nature Communications
Few-femtosecond time-resolved study of the UV-induced dissociative dynamics of iodomethane
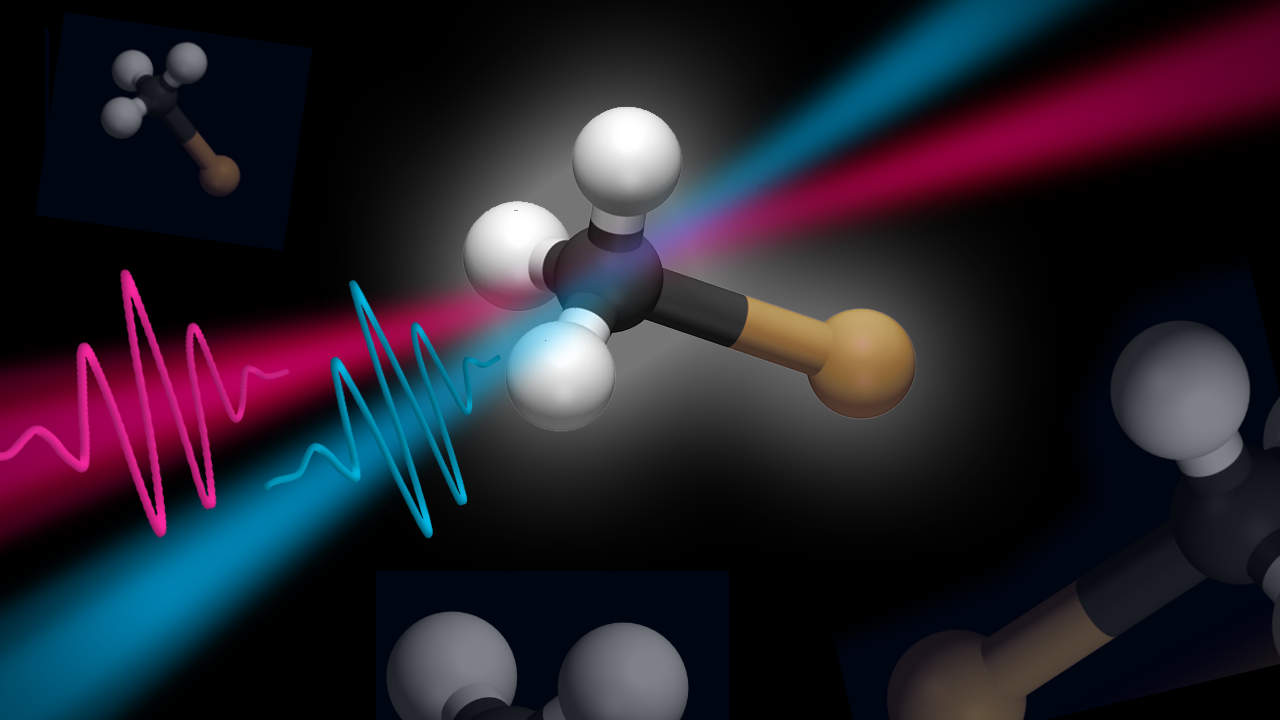
An ultrashort UV pulse causes photolysis of neutral iodomethane, which can be avoided if a second laser pulse arrives within 5 femtoseconds after photoexcitation.
Credit: Nicoletta Calegari
Abstract: Ultraviolet (UV) light that penetrates our atmosphere initiates various photochemical and photobiological processes. However, the absence of extremely short UV pulses has so far hindered our ability to fully capture the mechanisms at the very early stages of such processes. This is important because the concerted motion of electrons and nuclei in the first few femtoseconds often determines molecular reactivity. Here, we investigate the dissociative dynamics of iodomethane following UV photoexcitation, utilizing mass spectrometry with a 5 fs time resolution. The short duration of the UV pump pulse (4.2 fs) allows the ultrafast dynamics to be investigated in the absence of any external field, from well before any significant vibrational displacement occurs until dissociation has taken place. The experimental results combined with semi-classical trajectory calculations provide the identification of the main dissociation channels and indirectly reveal the signature of a conical intersection in the time-dependent yield of the iodine ion. Furthermore, we demonstrate that the UV-induced breakage of the C-I bond can be prevented when the molecule is ionized by the probe pulse within 5 fs after the UV excitation, showcasing an ultrafast stabilization scheme against dissociation.
More information can be found in the DESY press release.
The online publication can be found here.